Model: LX 1000
EAN: 4897080 000008
FDA Listed Device Number: D316762
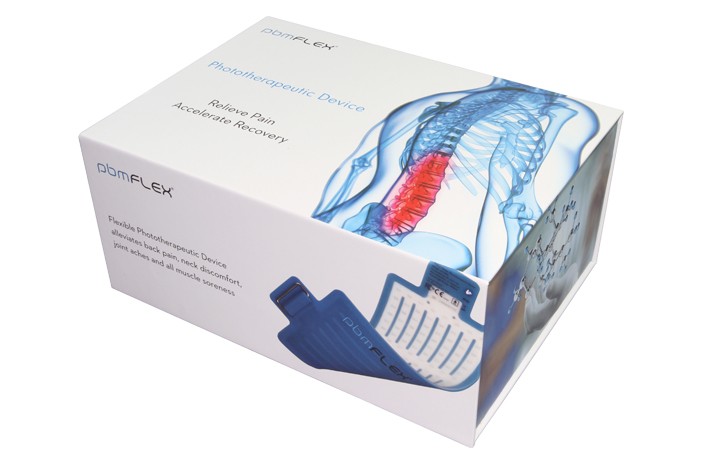
PBMFLEX® flexible Phototherapeutic Device alleviates back pain, neck discomfort, joint aches and muscle soreness. The device is developed with photobiomodulation (PBM) or low-level light therapy (LLLT) technology to maintain health – Uses the body’s natural processes to heal.
Product Features
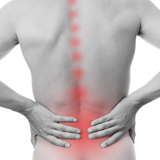
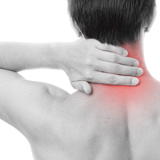
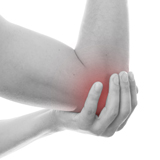
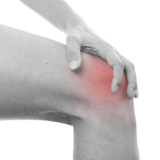
 PBMFLEX® Phototherapeutic Device LX 1000 alleviates back pain, neck discomfort, joint aches and muscle soreness.
PBMFLEX® Phototherapeutic Device LX 1000 alleviates back pain, neck discomfort, joint aches and muscle soreness.
Photobiomodulation (PBM) or low-level light therapy (LLLT) technology to maintain health - Uses the body’s natural processes to speed up recovery.
880 nm monochromatic LED light stimulates the damaged cells repairing them to their natural state.
Symptoms to treat
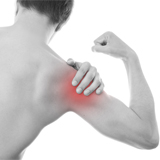
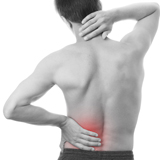
Pain or aches in joints caused by rheumatoid arthritis (RA) and osteoarthritis (OA), muscle aches and soreness caused by inflammation.
* It is important to note that PBMFLEX HEALTH recommends that you always consult a specialist or your primary physician to be correctly diagnosed.
Suitable for the most part of the body
PBMFLEX® flexible Phototherapeutic Device is suitable to be attached on lower back, neck, shoulder, elbow, wrist, knee, popliteus and ankle, etc. to treat pain or stiffness.
Simple and convenient to use
User friendly controller offers your daily treatment simple and convenient. You can use PBMFLEX® Phototherapeutic Device at home and work, when watching TV or sleeping or sitting.
Flexible and durable material
PBMFLEX® Phototherapeutic Device is made of flexible and durable material, hygienic silicone, with innovative design for the most comfort of use. The Device is designed to be attached onto the skin and held against the body with a soft strap.
Waterproof and easy clean
PBMFLEX® Phototherapeutic Device is waterproof and can be cleaned with damp cloth, with water and the most popular household cleaners in the market, can also be sterilized with the most popular household disinfectants in the market.
Specifications
Device Specifications |
||
|
Photon Wavelength: |
880 nm |
|
|
Photon Energy: |
1.8 mW/cm2 (at Standard energy level) |
|
|
Classification: |
Risk Group 1 |
|
|
LED Emitting Area: |
22.7 cm x 9.8 cm |
|
|
Pad Weight: |
175 g |
|
|
Pad Material: |
Silicone |
|
|
Device Input: |
10V DC, 1.5 A |
|
|
Power Supply Class: |
Class II |
|
|
Power Supply Input: |
100 – 240V AC, 50 – 60 Hz |
|
|
Power Cord Length: |
2 m |
|
|
Velcro® Straps: |
1 for waist size 28” – 42” |
|
|
Operating Environment: |
Temperature 10 °C – 35 °C |
|
Safety Testing and Certifications
Tests Passed and Standards Met
– EN 60601-1:2006+A1:2013
Medical electrical equipment – Part 1: General requirements for basic safety and essential performance
– EN 60601-1-11:2010
Medical electrical equipment – Part 1-11: General requirements for basic safety and essential performance – Collateral Standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment
– EN 60601-1-2-57:2011
Medical electrical equipment – Part 2: Particular requirements for the basic safety and essential performance of non-laser light source equipment intended for therapeutic, diagnostic, monitoring and cosmetic/aesthetic use
– EN 60601-1-6:2010+A1:2015
Medical electrical equipment – Part 1-6: General requirements for safety – Collateral Standard: Usability
– EN 62366:2008+A1:2015
Medical devices – Application of usability engineering to medical devices
– EN 60601-1-2:2007
Medical electrical equipment – Part 1-2: General Requirements for basic safety and essential performance – Collateral standard: Electromagnetic compatibility (EMC)
– CFR 47 FCC PART 15 SUBPART B:2014
Electromagnetic Interference (EMI)
– EN ISO 10993-1:2009/AC:2010
Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process
– EN ISO 10993-5:2009
Biological evaluation of medical devices – Part 5: Tests for in vitro cytotoxicity
– EN ISO 10993-10:2013
Biological evaluation of medical devices – Part 10: Tests for irritation and skin sensitization
– EN 62471-5:2008
Photobiological safety of lamps and lamp systems
FDA Registration and Listing
Establishment Registration Number: 3014331305
Listed Device Number: D316762
FCC Certification
Certificate issued by SGS-CSTC
In conformity with CFR 47 FCC PART 15 SUBPART B:2014
CB Certification
Certificate issued by SGS Fimko
In conformity with IEC 60601-1:2005, IEC 60601-1:2005/AMD1:2012, IEC 60601-1-11:2010, IEC 60601-2-57:2011, IEC 60601-1-6:2010 and IEC 60601-1-6:2010/AMD1:2013
Certifications in Progress
ISO 13485 Quality Management System
Medical CE